The PEM electrolyzer is a crucial technology in the realm of renewable energy production. By utilizing proton exchange membrane technology, these electrolyzers efficiently split water into hydrogen and oxygen, offering a clean energy solution. This process enables the storage of excess renewable energy generated from sources like solar or wind power. As the demand for sustainable energy sources continues to rise, PEM electrolyzers play a vital role in facilitating the transition towards a greener future.
Unraveling the Science Behind PEM Electrolyzers
Electricity Conversion
PEM electrolyzers use electricity and water to produce hydrogen and oxygen through a chemical reaction.
The process involves splitting water molecules into their constituent elements, hydrogen and oxygen, using an electrical current. This conversion occurs within the PEM electrolyzer, where electricity is passed through water to initiate the electrolysis process.
Role of Solid Polymer Electrolyte Membrane
The solid polymer electrolyte membrane in PEM electrolyzers plays a crucial role in facilitating the conversion of water into hydrogen and oxygen. It acts as a conductor for protons while simultaneously insulating the electrodes.
This membrane is essential for separating the two electrodes within the electrolyzer and ensuring that only protons can pass through it during the electrolysis process. By conducting protons, the membrane enables the movement of ions necessary for generating hydrogen gas at one electrode and oxygen gas at the other.
Enthalpy Requirements
In PEM electrolysis, a specific amount of enthalpy is required for water decomposition to occur successfully. This enthalpy represents the energy needed to break down water molecules into their individual components, hydrogen and oxygen.
Water decomposition involves overcoming the bond strength between hydrogen and oxygen atoms within each water molecule. The enthalpy input during this process ensures that sufficient energy is provided to separate these atoms effectively, leading to the production of hydrogen gas at one electrode and oxygen gas at the other.
Core Reactions in PEM Electrolysis
Anode Reaction
In a PEM electrolyzer, the anode reaction involves the Oxygen Evolution Reaction (OER). This crucial process occurs at the anode, where water molecules split into oxygen gas, protons, and electrons. The equation for this reaction is 2H₂O → O₂ + 4H⁺ + 4e⁻.
The products of the OER are vital for generating oxygen gas, which can be used in various industrial applications such as metal cutting and welding. The protons produced during this reaction play a significant role in forming hydrogen gas at the cathode.
Cathode Reaction
At the cathode of a PEM electrolyzer, the Hydrogen Evolution Reaction (HER) takes place. During this process, protons and electrons combine to form hydrogen gas. The equation representing this reaction is 2H⁺ + 2e⁻ → H₂.
The output of the HER is hydrogen gas, which holds immense potential as a clean energy source for fuel cells and transportation applications. By harnessing this reaction effectively, PEM electrolyzers can contribute to reducing carbon emissions and promoting sustainable energy solutions.
Significance of Reactions
The core reactions in PEM electrolysis play a crucial role in producing hydrogen and oxygen, two essential elements with diverse industrial and environmental applications. Hydrogen, generated through the cathode reaction, serves as a clean fuel alternative with zero greenhouse gas emissions when utilized in fuel cells or vehicles.
Moreover, oxygen produced via the anode reaction is indispensable for supporting combustion processes and sustaining life through various medical applications like respiratory therapies. Understanding and optimizing these reactions are paramount for enhancing the efficiency and scalability of PEM electrolysis technology.

Advantages Over Other Electrolyzer Types
High Current Densities
PEM electrolyzers excel in operating at high current densities, making them cost-effective compared to other types. This efficiency stems from the utilization of polymer electrolyte membranes that facilitate rapid ion transport, enhancing overall performance.
The ability to sustain high current densities allows PEM electrolysis to produce hydrogen more efficiently, meeting the increasing demand for clean energy sources. By optimizing electrode designs and materials, these systems can achieve enhanced productivity while minimizing operational costs.
High Gas Purity
Maintaining high gas purity is crucial in PEM electrolysis for ensuring storage safety and supporting the utilization of hydrogen in fuel cells. The cathode and anode electrodes, along with a specialized catalyst, play a vital role in promoting gas purity by facilitating the separation of oxygen and hydrogen molecules.
Incorporating advanced catalysts within PEM electrolyzers enhances their efficiency in generating high-purity hydrogen gas, suitable for various industrial applications. This purity level is essential for preventing contamination issues during hydrogen storage and subsequent usage in fuel cells.
Understanding Voltage and Efficiency Losses
Factors Contributing to Losses
Ohmic losses occur due to resistance in the electrolyte and electrodes, leading to voltage drops. Faradaic losses, on the other hand, result from inefficiencies in the conversion of electrical energy into chemical bonds.
Efficiency in PEM electrolyzers is impacted by current densities and cell voltage. Higher current densities can cause higher ohmic losses, reducing overall efficiency.
Optimizing Voltage for Efficiency
By optimizing the operating voltage of a PEM electrolyzer, it's possible to enhance electrical efficiency. Lowering cell voltages can minimize energy losses due to overpotential effects, ultimately improving the overall efficiency of the electrolysis process.
Maintaining an optimal balance between cell voltage and current density is crucial for maximizing efficiency while minimizing energy consumption. This balance ensures that the electrolyzer operates at peak performance levels.
Strategies for Minimizing Losses
To reduce efficiency losses, manufacturers focus on improving the durability and performance of PEM electrolyzers. Enhanced materials selection, advanced catalysts, and optimized system designs play key roles in minimizing losses and enhancing overall efficiency.
Implementing efficient heat management systems helps mitigate heat-related issues that can affect performance. By effectively managing heat energy within the system, PEM electrolyzers can maintain optimal operating conditions for improved efficiency.
Incorporating smart control systems that regulate factors such as temperature, pressure, and flow rates can further optimize the performance of PEM electrolyzers. These systems ensure consistent operation and maximize energy conversion rates.
Exploring Hydrogen Compression Techniques
Mechanical Compression
Mechanical compression is a widely used technique for compressing produced hydrogen. It involves using pistons or diaphragms to increase the pressure of hydrogen gas. This method is efficient but can be energy-intensive.
Cryogenic Compression
Cryogenic compression utilizes low temperatures to condense gases into a liquid state, reducing their volume for storage. This technique is effective in achieving high levels of compression for hydrogen. However, it requires significant energy for cooling.
Solid-state Storage
id-state storage involves adsorbing hydrogen molecules onto solid materials like activated carbon. While this method offers a safe and compact way to store hydrogen, it can be challenging to release the gas when needed.
Importance of Hydrogen Compression
Efficient hydrogen compression is crucial for storing and transporting produced hydrogen effectively. It allows for maximizing the amount of hydrogen that can be stored in a given space, enabling its use in various applications.
Efficiency and Challenges
Different compression methods vary in terms of efficiency and challenges. Mechanical compression is efficient but energy-intensive, while cryogenic compression offers high levels of compression at the cost of significant energy consumption. Solid-state storage provides safety benefits but faces challenges in releasing stored hydrogen efficiently.
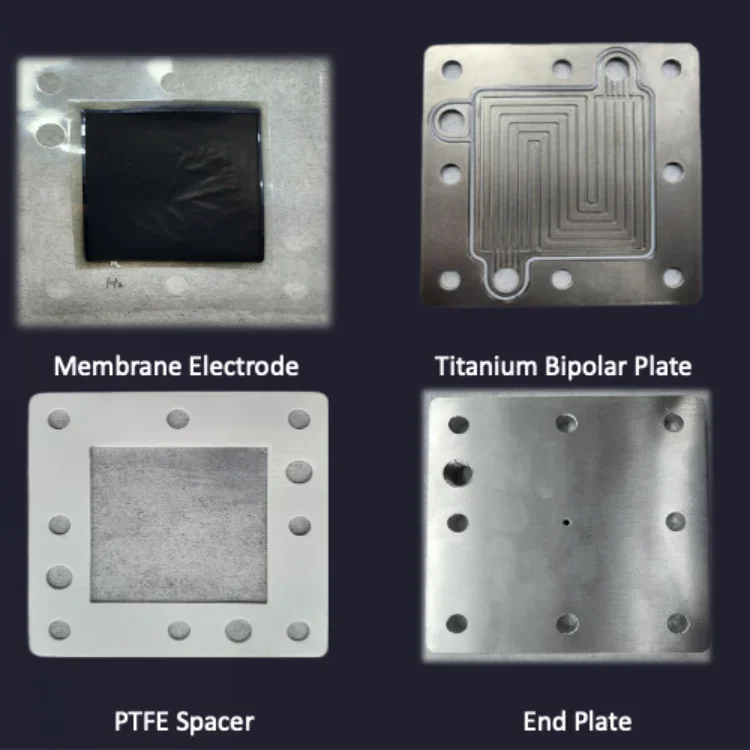
Navigating System Operations of PEM Electrolyzers
Key Components
PEM electrolyzers consist of essential components such as membrane electrode assemblies (MEAs), bipolar plates, and catalysts. These components work together to facilitate the electrolysis process efficiently.
The MEA, a crucial part of the electrolyzer, comprises a proton exchange membrane sandwiched between two electrodes. The bipolar plates ensure uniform distribution of reactants across the cell, enhancing performance.
Process Flow
The operation of a PEM electrolyzer begins with the application of an electric current to the MEA. This electrical input initiates the separation of water molecules into hydrogen and oxygen.
As electricity passes through the MEA, water molecules undergo electrolysis, producing hydrogen at the cathode and oxygen at the anode. The separated gases are then collected for further applications.
Control Mechanisms
To optimize performance and safety, PEM electrolyzers employ sophisticated control mechanisms. Pressure sensors monitor gas pressure levels within the system, ensuring stable operation.
Moreover, temperature sensors regulate heat levels during electrolysis to prevent overheating and maintain optimal working conditions. These sensors play a vital role in safeguarding equipment integrity.
Monitoring Systems
In addition to control mechanisms, monitoring systems are integrated into PEM electrolyzers for real-time assessment of operational parameters. Flow meters track gas flow rates, enabling precise measurement and control.
Furthermore, voltage sensors monitor electrical output to ensure consistency and efficiency throughout the electrolysis process. These monitoring systems enhance overall system reliability.
Maximizing PEM Efficiency
Maintenance Tips
Regular maintenance is crucial for maximizing the efficiency of PEM electrolyzers. Cleaning the electrolyzer components and ensuring proper sealing can prevent leaks and maintain optimal performance. Monitoring the electrode condition and replacing them when necessary can significantly improve efficiency.
Integration with Renewable Energy
Integrating PEM electrolyzers with renewable energy sources like solar or wind power presents a promising opportunity for sustainable hydrogen production. By utilizing renewable energy, the overall carbon footprint of hydrogen production can be reduced, contributing to environmental conservation efforts.
Monitoring Performance
Continuous monitoring of PEM electrolyzer performance is essential to ensure consistent operation and efficiency. Tracking key parameters such as temperature, pressure, and current density can help identify issues early on and optimize system settings for improved productivity.

Final Remarks
The exploration of PEM electrolyzers has shed light on their intricate science, core reactions, advantages over other types, voltage considerations, hydrogen compression methods, system operations, and efficiency optimization. These sections collectively underscore the pivotal role PEM electrolyzers play in the realm of hydrogen production. Understanding their inner workings and operational nuances is crucial for maximizing their efficiency and harnessing the full potential of this cutting-edge technology.
For those delving into the realm of hydrogen production or seeking to enhance their knowledge of PEM electrolyzers, a deeper dive into these facets is highly recommended. By grasping the intricacies discussed herein and implementing best practices, individuals and industries can propel towards a greener future powered by hydrogen. The journey towards sustainable energy solutions begins with a comprehensive understanding of technologies like PEM electrolyzers.
Frequently Asked Questions
What are PEM electrolyzers?
PEM electrolyzers, or Proton Exchange Membrane electrolyzers, are devices that use an electrolyte membrane to split water into hydrogen and oxygen through an electrochemical process.
How do PEM electrolyzers differ from other types of electrolyzers?
PEM electrolyzers offer advantages such as higher efficiency, faster response times, and compact size compared to alkaline or solid oxide electrolyzers.
What are the core reactions involved in PEM electrolysis?
The main reactions in PEM electrolysis involve the splitting of water molecules into hydrogen ions (protons), electrons, and oxygen gas. The hydrogen ions migrate through the membrane, while the electrons form the electrical current.
How can voltage and efficiency losses be understood in PEM electrolysis?
Voltage and efficiency losses in PEM electrolysis occur due to factors like resistance in the cell components, activation overpotentials at electrodes, and mass transport limitations within the cell.
What techniques are used for hydrogen compression in PEM electrolysis systems?
Hydrogen compression techniques in PEM systems include methods like mechanical compression using pistons or diaphragms, as well as advanced technologies like cryogenic cooling or adsorption-based systems.








